Cord blood collection
More than 450 medical professionals have collected cord blood for our clients at over 50 maternity hospitals throughout the Russian Federation. CryoCenter has provided extensive educational resources for obstetricians and midwives. If necessary, medical personnel may be additionally trained in cord blood collection by our specialists on the spot.
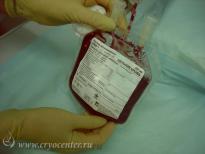
CryoCenter provides to expectant parents the collection kit which contains all the items the doctor or midwives will need for safe and effective cord blood collection. The kit is admissible into a sterile field for use in Caesarian sections.
Without any delay after collection, the isothermal container with cord blood and accompanying medical documentation is delivered to CryoCenter’s state-of-the-art laboratory for processing.
The developed in CryoCenter logistics system allows us to acquire samples even from the innermost regions of Russia in a time delay not exceeding 24 hours.
Cord blood processing



The amount of stem cells in cord blood constitutes about 0.1-1 percents from all leukocytes. Red blood cells and other mature cells are useless for transplantation. During the cord blood step-by-step separation procedure we remove erythrocytes and the excess of plasma.
Cell separation is performed by qualified personnel in a specialized “clean-room” facility under fully sterile conditions. All the consumables are single-use and are supplied by world leading manufacturers.
CryoCenter uses an optimal combination of “manual” and automated cell separation techniques. These allow stem cells to be extracted with negligible losses from cord blood samples of various volumes and efficiently prepared them for long-term cryogenic storage.
The expertise of our staff and state-of-the-art innovations in cell technologies allow us to preserve the level of cell viability higher than in many other facilities. In the most of cases the viability isolated stem cells exceeds 99.9 percent.
ALL the samples released by CryoCenter for clinical application have been proved viable when thawed – the best validation of our processing methods.
Freezing stem cells

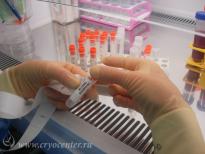

After a cell suspension in autologous plasma is mixed with cryoprotectant solution, the cells are distributed into special cryogenic containers which are marked with a unique digital code and a barcode. Each container is placed into a protective envelop which guarantees the safety of the contents and of identifying information. Depending on the amount of cells, the material can be packed into single or several containers: this allows the patient to use for therapy the necessary amount of cells and keep the rest for a future need, taking into account the rapid development of new cell technologies.
Each sample of stem cells is accompanied by “satellites” – small cell aliquots which can be used to perform additional tests before clinical use.
CryoCenter practices only programmable freezing of cell material. This provides optimal cooling rates and maximal viability of the isolated cells.
What testing is performed by CryoCenter to characterize cord blood stem cells?

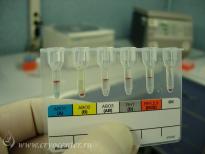
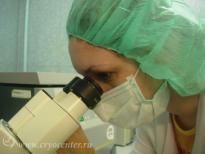
In addition to all the appropriate tests performed during pregnancy, the collected cord blood is screened for markers of dangerous transmissible diseases – HIV-1/2, hepatitis B and C, Human T-lymphotropic virus, cytomegalovirus, toxoplasmosis, and syphilis.
During the separation procedure every sample of cord blood and isolated stem cells are characterized by:
- total nucleated cell count;
- blood group and Rh-typing;
- flow-cytometric measurement of relative and absolute content of stem cells;
- viability of cells prior to freezing;
- sterility test.
Only those samples which have been recognized as “clean” are admitted into the CryoCenter’s main storage area.
Stem cell storage


Cord blood stem cells are stored in cryogenic vaults filled with liquid nitrogen called Dewars at a temperature of -196 ˚C. In these conditions cells can retain their viability and biological activity for a virtually unlimited period of time.
The storage area is equipped with an autonomous power generator and is under 24-hour surveillance. Each dewar is monitored continuously for liquid nitrogen levels and temperature.
The stem cell storage facility has limited access and additional security precautions.
Unique computer database
The results of each manipulation are logged in hard-copy forms and registered into a computer database under a unique digital code. The use of specialized software designed especially for CryoCenter prevents mistakes during sample identification and allows us to know the complete history of the sample and its precise location in the storage vessel.
Laboratory security
CryoCenter’s laboratory features backup power supplies, computer monitoring systems and a system that maintains constant contact with personnel whilst monitoring the laboratory and cryogenic vaults. Our storage facilities are under twenty-four-hour security and surveillance and the entire area is alarmed and monitored.
