Dear Expectant Parents!
For more than 14 years the Cord Blood Bank "CryoCenter" provides qualified medical services for the cryogenic storage of umbilical cord blood cells. More than 20,000 of our fellow citizens have entrusted us to store the most valuable biological insurance for their children. But CryoCenter is not just a Cord Blood Bank – together with experts in various fields of medicine we are engaged in scientific research, organize and conduct clinical trials, develop and successfully put into practice the newest treatment technologies – cell therapy.
Today we are pleased to announce that after the successful completion of all necessary studies CryoCenter is ready to provide you a new biomedical service: cryogenic storage of umbilical cord tissue and isolated from it mesenchymal stromal (stem) cells (MSCs).
What MSCs are, what are the realities and perspectives of their application in medicine, you will learn from this publication.
MESENCHYMAL STEM CELLS:
a new era of regenerative medicine
Since the early 1970s, when the Russian scientist Alexander Fridenstein has described bone marrow "fibroblast’s colony forming units" (CFU-f), this unique cellular population has changed a few names. Until recently, the most popular among cell biologists and physicians was the term "mesenchymal stem cells" (MSCs) proposed in the 1990s by Arnold Caplan. Fifteen years later, by the initiative of the International Society for Cellular Therapy (ISCT) it was replaced by “multipotent mesenchymal stromal cells", retaining the familiar abbreviation MSCs.
All three terms denote a population of cells able to grow in the attached state and to differentiate (to mature, acquire properties) in the experimental conditions, at least in three directions: osteogenic, adipogenic, and chondrogenic, i.e. to give rise to cells of bone, adipose and cartilage tissue. In the recent years it has been demonstrated the ability of MSCs to participate in formation of other types of cells in our body: muscle, endothelial (lining the inner surface of blood vessels), cardiomyocytes (heart cells), hepatocytes (liver cells) and neurons.
Traditional and exotic sources of MSCs
The main source of MSCs for both experimental and clinical studies historically was the bone marrow. However, only 0.001 to 0.01 % of all cells present in bone marrow aspirate are able to attach to the plastic and to give clonal growth of fibroblastoid elements. In addition to bone marrow, more or less MSCs can be isolated from almost all ever studied human tissues: adipose tissue, amniotic fluid, skin, peripheral and cord blood and even menstrual blood and the pulp of fallen milk teeth. However, the isolation and successful propagation of MSCs from most of these exotic sources is generally difficult due to methodological complexity or very low contents of the desired cells.
Truly unbeatable sources of MSCs are umbilical cord and placenta.
The umbilical cord – a unique source of MSCs
During the pregnancy, two organisms – the mother and fetus – are connected by the umbilical cord: rope-like structure with a diameter of approximately one and a length of about 50 centimeters. The umbilical cord contains three blood vessel (two arteries and one vein) that are enclosed in gelatinous extracellular substance (Wharton’s jelly), covered outside with a dense wrap. According to available literature data, all the "compartments" of the umbilical cord contain MSCs.
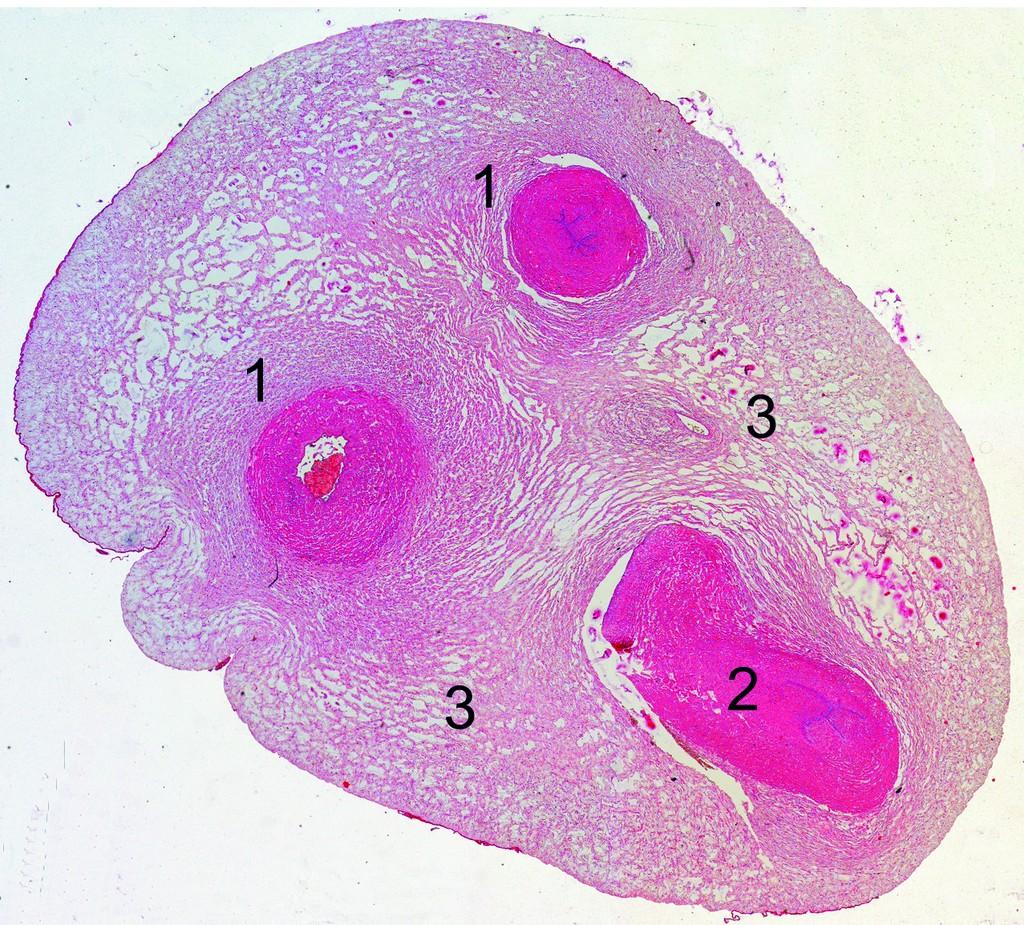
Cross-section of the human umbilical cord.
1 – umbilical artery, 2 – umbilical vein, 3 – Wharton’s jelly.
(Photo courtesy of Dr. I. Arutunyan).
Compared to adult cells, umbilical cord MSCs possess the highest proliferative potential (ability to replicate) and biological activity, making them one of the main candidates for use in regenerative medicine and cell therapy. These cells can use all three of the known mechanisms of repair: direct differentiation into the desired type of cells, paracrine and immunomodulatory actions (see below). Although umbilical cord MSCs are not as multipotent as embryonic or induced pluripotent stem (iPS) cells, they have another distinct advantage – the lack of capacity to form malignancy and development of teratomas (embryonic tumors) in experimental conditions. Moreover, as in the case of umbilical cord blood cells, their preparation does not cause any ethical, religious, medical or other problems and the harvesting of the umbilical cord tissue is carried out after the birth of a child and has no risk to himself or his mother.
Isolation and cultivation of umbilical cord MSCs
Since the content of MSCs in tissues (including the umbilical cord) is relatively low, the further work with them implies the need for multiplication in vitro. For these purposes, dozens of protocols based on the use of commonly available commercial synthetic media with the addition of serum or cocktails of growth factors have been proposed. In a relatively short time (weeks – months), the cell cultivation allows to increase MSC quantity to the required (hundreds of millions) for clinical use.
Synthetic and secretory activity of MSCs
Due to the anatomical location and many functions, MSCs synthesize and secrete a number of component of extracellular matrix (fibronectin, collagen, laminin, proteoglycans) and soluble compounds. MSCs produce growth factors, cytokines and chemokines directly involved in maintaining the normal functioning of organs and tissues, including interleukins (IL) -1α and -1β, IL-6, IL-7, IL-8, IL-11, IL-14 and IL-15, macrophage (M-CSF) and granulocyte-macrophage (GM-CSF) growth factors, stem cell factor (SCF), thrombopoietin and hepatocyte growth factor (HGF). Some of these compounds are produced spontaneously, others in response to the activation. Together and individually, these factors are involved in maintenance of normal homeostasis and the processes of regeneration (restoration) of damaged organs and tissues.
Immunomodulatory properties of MSCs
The ability of MSCs to exert anti-proliferative, immunomodulatory and anti-inflammatory effects in various pathological conditions has recently attracted more and more attention. MSCs themselves are weakly immunogenic and do not cause rejection by the recipient's body. Lack on their surface of some molecules gives grounds for the use in therapeutics of allogeneic (donor’s origin) cells. Such immunosuppressive properties of MSCs are realized by participation of produced soluble factors ("paracrine" mechanism of action) and/or direct intercellular contacts. And those and others can dramatically affect the course of physiological and pathological processes by changing properties of the cellular microenvironment from pro- to anti-inflammatory.
Biological markers of MSCs
Currently, there is no unique token that uniquely tell about the ownership of the cells to category “MSC”. Like any other cells in the body, MSCs are carrying on their surface hundreds of proteins related to the execution of particular functions. Among them are adhesion molecules responsible for cell-to-cell interaction, receptors and biologically active substances (cytokines and growth factors), proteins that mediate the recognition of "friend or foe", i.e. regulators of the immune system, and many others. Most of them are united under the term "cluster of differentiation" (CD) in a specific nomenclature.
According to the recommendations of the International Society for Cellular Therapy, obligatory for the MSCs markers include such as CD90, CD73 and CD105, as well as human leukocyte antigens of the 1st type (HLA-I). Besides these, MSCs contain on their surface CD13, CD29 and CD44, CD54, CD71, and (rarely) CD106. Concurrently, MSCs do not contain the known markers of blood cells, hematopoietic or endothelial progenitor cells, such as CD34, CD45, CD133 and HLA-DR. This "phenotype" of MSCs is usually used in the preparation of the Passport for the cellular product intended for research or clinical use.
The therapeutic potential of MSCs
Many of the characteristics of MSCs, including the ability to differentiate into various cell types in vitro and in vivo, are making them very attractive from the point of view of possible use for cell therapy of a wide spectrum of acquired and hereditary diseases. After systemic (intravenous, intra-arterial) and local (directly into the target tissue) administration MSCs have an ability to "homing" and long survival in various organs and tissues of the recipient. In addition, being administered into the blood system, these cells are able to purposefully migrate into sites of injury and participate in repair that was convincingly demonstrated in models of bone fracture, cerebral ischemia and myocardial infarction. There are indications of attempts to use MSCs in the treatment of articular cartilage defects.
The subsequent experimental and clinical studies conducted on a limited number of patients, have proved the possibility of using MSCs or their mixture with hematopoietic stem cells in ischemic limbs and myocardium, non-healing chronic skin wounds, etc. Encouraging results have been achieved in the treatment of neurological and cardiac diseases. Studies have shown that MSC transplantation may be effective in ischemic stroke, traumatic brain disease and spinal cord injury, myocardial infarction. Other examples of a successful application of MSCs can be seen in the reports confirming the accelerated recovery of hematopoiesis after transplantation of hematopoietic stem cells and positive effect in the treatment of severe forms of acute and chronic “graft-versus-host disease” (GVHD), also associated with a pronounced "immunomodulatory" properties of MSC. Several reports demonstrate the successful use of MSC for the treatment of diseases of other organs: lungs, muscles and kidneys.
Cryogenic storage of MSCs
Like most other types of human cells, umbilical cord MSCs can for decades to preserve their biological properties in cryogenic conditions (in liquid nitrogen). Subsequently, cells can be successfully thawed without loss of quality or used (depending on quantity) either for clinical use or for additional propagation with the same purpose.
Conclusion
The successes achieved in the study of MSC biology in vitro and in experimental models in vivo, could not fail to attract the attention of medical practitioners. The list of clinical trials, officially declared in the different countries of the world includes hundreds and is growing steadily, as well as the number of reports indicating the effectiveness of cell therapy in various pathological conditions. With the discovery and successful isolation of MSCs from different post-embryonic tissues, including umbilical cord tissue, began a new era of cell therapy. We want to believe that in the foreseeable future these cells will occupy a worthy place and bring practical medicine to a new level – the level of regenerative medicine.
TECHNOLOGY
Unique methodologies, developed and tested by specialists of CryoCenter allow you to keep two types of biological materials derived from umbilical cord tissue:
- isolated and characterized MSCs, which (if necessary) can be additionally propagated for further use (among other, it confirms that before freezing the original umbilical cord tissue contained MSCs in sufficient number and with high viability);
- the fragment of umbilical cord tissue itself, which after many years can be thawed and used for cell separation in conditions for that time optimal.
Both methods showed high efficiency, and the results (along with others) were published on the pages of scientific periodicals.
Harvesting the umbilical cord tissue
Immediately after the collection of umbilical cord blood, the middle part of the umbilical cord is clumped by two additional clips to prevent infection. A fragment of the umbilical cord with a length of 10-15 cm is excised and placed in a sealed transport container. The container will be marked with the same unique code as umbilical cord blood, and transported to the laboratory of CryoCenter under isothermal conditions.



Preparation of umbilical cord tissue for cryogenic storage
In a sterile environment, obtained umbilical cord is treated with a disinfectant solution and cut with a sterile tool into small pieces which are washed from the remnants of blood with a sterile saline solution. The resulting material is distributed in a pre-labeled sterile cryovials and a solution of cryoprotectant (similar to that for the cryopreservation of umbilical cord blood cells) is added. The process of programmable freezing of umbilical cord tissue and all subsequent procedures do not differ from the protocols of freezing cord blood cells.
Isolation of MSCs
Inside the umbilical cord tissue MSCs are surrounded by a dense extracellular substance (matrix). To separate them, the use of a special enzyme solution is required. Then the resulting cell suspension is washed and diluted in culture medium, seeded into sterile culture flasks and placed in an incubator, supporting the necessary temperature, gas composition and humidity. Two-three weeks later, when the cells had time to multiply to a specified amount, they will be distributed in new bottles: a part of them will be used to carry out the necessary tests (including, phenotyping); other cells are intended for subsequent storage. Approximately after another 2 weeks, the cells are removed from vials, transferred to cryotubes and frozen. According to the test results (cell number, viability, sterility, phenotype) a Passport is given for each sample.



The unique laboratory complex
All works on the isolation, cultivation and preparation for cryogenic storage of umbilical cord cells and tissue are conducted by qualified personnel (cell biologists) in certified GMP-compliant laboratory furnished with the newest equipment. The laboratory is located in an isolated sector of CryoCenter’s manufacturing complex and uses all of its existing technological capabilities (analytical laboratory, programmable freezing, cryogenic storage, laboratory of flow cytometry, electronic database, etc.).
The MSC phenotyping
Flow cytometry – methodological procedure that allows making a portrait of even a single cell by the detection on its surface of specific markers responsible for the performance of a particular function. As in the case of umbilical cord blood cells, MSC phenotyping is carried out using a special device – a flow cytometer permitting to characterize the cell suspension by 5 to 7 parameters.


Cryogenic storage
Vials with umbilical cord tissue fragments and pre-expanded MSCs are stored in the same conditions as cells from umbilical cord blood: in cryogenic (Dewar) vessels equipped with sensors for temperature and level of liquid nitrogen. The time of cells/tissue storage may be as long as many decades and is practically unlimited.
Experience, proven over the years
CryoCenter works closely with many research laboratories and institutes. Among them – the Laboratory of Human Stem Cells of the National Cardiology Research Center, Ministry of Health of the Russian Federation – its scientists in 2003 the world's first described the presence in the umbilical cord tissue of "MSC-like" cells, developed a method of isolation, and described their characteristic. In subsequent years, the laboratory’s and CryoCenter’s investigators have published dozens of joint research papers and presentations concerning stem cell biology and their practical application.
